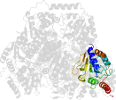
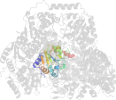
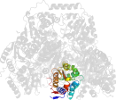
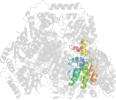
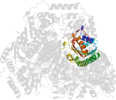Lineage for d1ozha2 (1ozh A:7-187)
- Root: SCOPe 2.08

Class c: Alpha and beta proteins (a/b) [51349] (148 folds) 
Fold c.36: Thiamin diphosphate-binding fold (THDP-binding) [52517] (1 superfamily)
3 layers: a/b/a; parallel beta-sheet of 6 strands, order 213465
Superfamily c.36.1: Thiamin diphosphate-binding fold (THDP-binding) [52518] (9 families) 
there are two different functional modules of this fold: pyridine-binding (Pyr) and pyrophosphate-binding (PP) modules
two Pyr and two PP modules assemble together in a conserved heterotetrameric core that binds two THDP coenzyme molecules
Family c.36.1.5: Pyruvate oxidase and decarboxylase Pyr module [88724] (8 proteins)
the N-terminal, Pyr module is separated from the C-terminal, PP module by an alpha/beta domain of Rossmann-like topology
automatically mapped to Pfam PF02776
Protein Catabolic acetolactate synthase [102328] (1 species) 
Species Klebsiella pneumoniae [TaxId:573] [102329] (3 PDB entries) 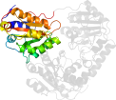
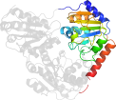
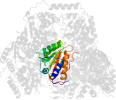
Domain d1ozha2: 1ozh A:7-187 [93834]
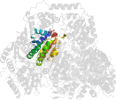
Other proteins in same PDB: d1ozha1, d1ozha3, d1ozha4, d1ozhb1, d1ozhb3, d1ozhb4, d1ozhc1, d1ozhc3, d1ozhc4, d1ozhd1, d1ozhd3
complexed with he3, mg, peg, pge, po4
Details for d1ozha2
PDB Entry: 1ozh (more details), 2 Å
PDB Description: The crystal structure of Klebsiella pneumoniae acetolactate synthase with enzyme-bound cofactor and with an unusual intermediate.
PDB Compounds: (A:) Acetolactate synthase, catabolicSCOPe Domain Sequences for d1ozha2:
Sequence, based on SEQRES records: (download)
>d1ozha2 c.36.1.5 (A:7-187) Catabolic acetolactate synthase {Klebsiella pneumoniae [TaxId: 573]}
vrqwahgadlvvsqleaqgvrqvfgipgakidkvfdslldssiriipvrheanaafmaaa
vgritgkagvalvtsgpgcsnlitgmatansegdpvvalggavkradkakqvhqsmdtva
mfspvtkyaievtapdalaevvsnafraaeqgrpgsafvslpqdvvdgpvsgkvlpasga
p
Sequence, based on observed residues (ATOM records): (download)
>d1ozha2 c.36.1.5 (A:7-187) Catabolic acetolactate synthase {Klebsiella pneumoniae [TaxId: 573]}
vrqwahgadlvvsqleaqgvrqvfgipgakidkvfdslldssiriipvrheanaafmaaa
vgritgkagvalvtsgpgcsnlitgmatansegdpvvalggavkradksmdtvamfspvt
kyaievtapdalaevvsnafraaeqgrpgsafvslpqdvvdgpvsgkvlpasgap
SCOPe Domain Coordinates for d1ozha2:
Click to download the PDB-style file with coordinates for d1ozha2.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1ozha2:
- d1ozha2 first appeared in SCOP 1.67
- d1ozha2 appears in SCOPe 2.07

 View in 3D
View in 3D