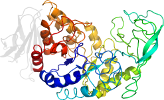
Lineage for d1ob0a1 (1ob0 A:394-483)
- Root: SCOPe 2.01

Class b: All beta proteins [48724] (174 folds) 
Fold b.71: Glycosyl hydrolase domain [51010] (1 superfamily)
folded sheet; greek-key
Superfamily b.71.1: Glycosyl hydrolase domain [51011] (5 families) 
this domain is C-terminal to the catalytic beta/alpha barrel domain
Family b.71.1.1: alpha-Amylases, C-terminal beta-sheet domain [51012] (22 proteins)
this domain follows the catalytic beta/alpha barrel domain
Protein Bacterial alpha-Amylase [51013] (9 species) 
Species Bacillus licheniformis [TaxId:1402] [51014] (8 PDB entries) 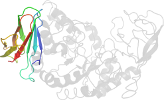
Domain d1ob0a1: 1ob0 A:394-483 [81256]
Other proteins in same PDB: d1ob0a2
complexed with ca, na
Details for d1ob0a1
PDB Entry: 1ob0 (more details), 1.83 Å
PDB Description: kinetic stabilization of bacillus licheniformis alpha-amylase through introduction of hydrophobic residues at the surface
PDB Compounds: (A:) alpha-amylaseSCOPe Domain Sequences for d1ob0a1:
Sequence; same for both SEQRES and ATOM records: (download)
>d1ob0a1 b.71.1.1 (A:394-483) Bacterial alpha-Amylase {Bacillus licheniformis [TaxId: 1402]}
yaygaqhdyfdhhdivgwtregdssvansglaalitdgpggakrmyvgrqnagetwhdit
gnrsepvvinsegwgefhvnggsvsiyvqr
SCOPe Domain Coordinates for d1ob0a1:
Click to download the PDB-style file with coordinates for d1ob0a1.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1ob0a1:
- d1ob0a1 first appeared in SCOP 1.63
- d1ob0a1 appears in SCOP 1.75
- d1ob0a1 appears in SCOPe 2.02
- d1ob0a1 appears in the current release, SCOPe 2.08

 View in 3D
View in 3D