Lineage for Class f: Membrane and cell surface proteins and peptides
- Root: SCOPe 2.08

Class f: Membrane and cell surface proteins and peptides [56835] (69 folds)
Folds:

f.1: Toxins' membrane translocation domains [56836] (5 superfamilies)
multi-helical domains of various folds which is thought to unfold in the membrane
f.3: Light-harvesting complex subunits [56917] (1 superfamily)
membrane all-alpha fold
f.4: Transmembrane beta-barrels [56924] (7 superfamilies)
not a true fold, gathers together transmembrane barrels of different (n,S)
annotated by the SCOP(e) curators as 'not a true fold'
f.5: Outer membrane efflux proteins (OEP) [56953] (1 superfamily)
subunit fold contains tandem repeat of alpha-beta hairpin-alpha(2) motif
trimeric fold contains barrel (n=12, S=18) formed by beta-hairpins, two from each subunit, and a bundle of helices with a channel running through it
f.6: Leukocidin-like [56958] (1 superfamily)
subunit fold contains beta-sandwich of Ig-like (greek-key) topology and a beta-ribbon arm that forms an oligomeric transmembrane barrel
f.7: Lipovitellin-phosvitin complex; beta-sheet shell regions [56967] (1 superfamily)
contains several large open beta-sheets
f.8: Aerolisin/ETX pore-forming domain [56972] (1 superfamily)
3 domains: (1) alpha+beta; (2&3) all-beta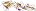
f.10: Viral glycoprotein, central and dimerisation domains [56982] (1 superfamily)
2 intertwined domains; all-beta and alpha+beta
f.12: Head and neck region of the ectodomain of NDV fusion glycoprotein [69921] (1 superfamily)
3 intertwined all-beta domains
f.13: Class A G protein-coupled receptor (GPCR)-like [81322] (1 superfamily)
core: up-and-down bundle of seven transmembrane helices tilted 20 degrees with respect to the plane of the membrane
f.14: Gated ion channels [81325] (2 superfamilies)
oligomeric transmembrane alpha-helical proteins
f.15: Small-conductance potassium channel [81328] (1 superfamily)
oligomeric transmembrane alpha-helical protein
f.16: Gated mechanosensitive channel [81331] (1 superfamily)
oligomeric transmembrane alpha-helical protein
f.17: Transmembrane helix hairpin [81334] (6 superfamilies)
two antiparallel transmembrane helices
f.18: F1F0 ATP synthase subunit A [81337] (1 superfamily)
core: up-and-down bundle of four transmembrane helices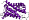
f.19: Aquaporin-like [81339] (1 superfamily)
core: 8 helices, 2 short helices are surrounded by 6 long transmembrane helices
f.20: Clc chloride channel [81341] (1 superfamily)
core: 18 transmembrane helices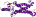
f.21: Heme-binding four-helical bundle [81344] (3 superfamilies)
core: four transmembrane helices, up-and-down bundle, binds one or two heme groups in between the helices
f.22: ABC transporter involved in vitamin B12 uptake, BtuC [81346] (1 superfamily)
multihelical; complex architecture with several transmembrane helices
f.23: Single transmembrane helix [81407] (42 superfamilies)
not a true fold
annotated by the SCOP(e) curators as 'not a true fold'
f.24: Cytochrome c oxidase subunit I-like [81443] (1 superfamily)
12 transmembrane helices in an approximate threefold rotational symmetric arrangement
f.25: Cytochrome c oxidase subunit III-like [81453] (1 superfamily)
core: 7 transmembrane helices organized into two bundles, one formed by the first two helices and the other by the rest
f.26: Bacterial photosystem II reaction centre, L and M subunits [81484] (1 superfamily)
five transmembrane helices forming a sheet-like structure
f.27: 14 kDa protein of cytochrome bc1 complex (Ubiquinol-cytochrome c reductase) [81525] (1 superfamily)
membrane-associated alpha-helical protein; no transmembrane helices
f.28: Non-heme 11 kDa protein of cytochrome bc1 complex (Ubiquinol-cytochrome c reductase) [81532] (1 superfamily)
membrane-associated alpha-helical protein; no transmembrane helices
f.29: Photosystem I subunits PsaA/PsaB [81559] (1 superfamily)
core:11 transmembrane helices
f.30: Photosystem I reaction center subunit X, PsaK [81564] (1 superfamily)
core: hairpin of two transmembrane helices
f.31: Photosystem I reaction center subunit XI, PsaL [81569] (1 superfamily)
core: three transmembrane helices, bundle
f.32: a domain/subunit of cytochrome bc1 complex (Ubiquinol-cytochrome c reductase) [81649] (1 superfamily)
core: three transmembrane helices, up-and-down bundle
f.33: Metal cation-transporting ATPase, transmembrane domain [81666] (1 superfamily)
core: multihelical; consists of three transmembrane regions of 2, 2 and 6 helices, separated by cytoplasmic domains
f.34: Mechanosensitive channel protein MscS (YggB), transmembrane region [82860] (1 superfamily)
oligomeric fold; 3 transmembrane helices per subunit
f.35: Multidrug efflux transporter AcrB transmembrane domain [82865] (1 superfamily)
12 transmembrane helices; duplication: the N- and C-terminal halves of the whole proteins are structurally similar
f.36: Neurotransmitter-gated ion-channel transmembrane pore [90111] (1 superfamily)
heteropentameric transmembrane alpha-helical protein; 4 transmembrane helices per subunit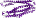
f.37: ABC transporter transmembrane region [90122] (1 superfamily)
multihelical; complex architecture with several transmembrane helices
f.38: MFS general substrate transporter [103472] (1 superfamily)
12 transmembrane helices; duplication: the N- and C-terminal halves are structurally similar
f.40: V-type ATP synthase subunit C [103485] (1 superfamily)
9 transmembrane helices
f.41: Preprotein translocase SecY subunit [103490] (1 superfamily)
10 transmembrane helices forming of a gated channel
f.42: Mitochondrial carrier [103505] (1 superfamily)
membrane all-alpha fold; 6-helical "barrel" with internal binding cavity
f.43: Chlorophyll a-b binding protein [103510] (1 superfamily)
membrane all-alpha fold; three transmembrane helices
f.44: Ammonium transporter [111351] (1 superfamily)
11 transmembrane helices; duplication: consist of 2 structural repeats of five helices each plus extra C-terminal helix
f.45: Mitochondrial ATP synthase coupling factor 6 [111356] (1 superfamily)
2 helices, hairpin
f.46: HlyD-like secretion proteins [111368] (1 superfamily)
consists of three domains: beta-barrel (res. 29-38,170-259; (50412)); barrel-sandwich hybrid (39-72,135-169; (51230)) and long alpha-hairpin (73-134; (46556))
f.47: VP4 membrane interaction domain [111378] (1 superfamily)
2 domains; d1: complexed all-beta fold; d2: coiled-coil (trimeric) helical region
f.48: OmpH-like [111383] (1 superfamily)
trimer; one subunit consists of an alpha/beta oligomerization subdomain [3-stranded parallel beta-sheet, order 213], and an antiparallel coiled coil
f.49: Proton glutamate symport protein [118214] (1 superfamily)
multihelical membrane protein; partial structural duplication in the C-terminal region; oligomeric state: trimer
f.50: Connexin43 [118219] (1 superfamily)
mostly unstructured; 2 helices, hairpin
f.51: Rhomboid-like [144090] (1 superfamily)
6 transmembrane helices
f.52: ATP synthase B chain-like [161059] (1 superfamily)
long single helix, part of the stator subcomplex
f.53: ATP synthase D chain-like [161064] (1 superfamily)
non-globular, mostly helical component of the stator subcomplex
f.54: SNF-like [161069] (1 superfamily)
12 transmembrane helices; duplication: consists of two structurally similar halves
f.55: Photosystem II antenna protein-like [161076] (1 superfamily)
6 transmembrane helices arranged in three antiparallel pairs (hairpins), segregated by cofactors; there can be insertions of different small subdomains in different exposed loops
f.56: MAPEG domain-like [161083] (1 superfamily)
4 helices, bundle; left-handed superhelix
f.57: MgtE membrane domain-like [161092] (1 superfamily)
5 transmembrane helices; bundle, right-handed twist
f.58: MetI-like [161097] (1 superfamily)
core: 5 transmembrane helices; flattened bundle
f.59: Cation efflux protein transmembrane domain-like [161110] (1 superfamily)
6 transmembrane helices, bundle
f.60: Anthrax protective antigen C-terminal-like [254103] (1 superfamily)
C-terminal 3 domains; II (226-487) is beta-sandwiches of gamma-crystallin like topology, similar to domain I; III (488-594) has a beta-grasp like fold; IV (595-735) has an Ig-like fold
f.61: Multidrug and toxic compound extrusion (MATE) transporter-like [310559] (1 superfamily)
12 transmembrane helices, in two bundles of 6
f.62: Diacylglycerol kinase (DgkA)-like [310569] (1 superfamily)
forms homotrimer; each monomer has 3 transmembrane helices and one amphiphilic helix
f.63: Claudin-like [345895] (1 superfamily)
4 transmembrane helices with 5-strand antiparallel beta-sheet, order 43215, on one side of membrane
f.64: Filovirus surface glycoprotein-like [418716] (1 superfamily)
pre-fusion conformation with complex fold including several alpha+beta domains
f.65: Vacuolar ATP synthase subunit a (also called I subunit) C-terminal domain [418717] (1 superfamily)
eight long membrane-embedded helices
f.66: Helical insert domain of RAG1 [418718] (1 superfamily)
bundle of 6 to 8 helices, somewhat irregular
f.67: Bestropin channel-like [418719] (1 superfamily)
four transmembrane helices; forms pentameric pore channel
f.68: Anoctamin (TMEM16)-like [418720] (2 superfamilies)
10 or 11 transmembrane helices, forms dimers
f.69: Perfringolysin N-terminal-like [418706] (1 superfamily)
twisted 4-strand antiparallel beta sheet, surrounded by other helices and strands, complex topology
f.70: Extracellular adhesion domain of SabA-like [418729] (1 superfamily)
club-like mostly helical structure; central region has similarity to 4-helix bundles such as bromodomains (a.29.6)
f.71: LYR proteins from mammalian respiratory complex I [418731] (1 superfamily)
three-helix bundle, with long extended loops that interact with other subunits
f.72: Antiporter-like subunits from respiratory complex I [418732] (1 superfamily)
core: 14 transmembrane helices; each has two groups of five helices are related to each other by pseudo two-fold symmetry, with the groups inverted with respect to the membrane
f.73: Non-antiporter membrane subunits from respiratory complex I [418733] (3 superfamilies)
Three subunits form a single 11-helix bundle at the interface between the hydrophilic domain and the antiporter-like subunits
More info for Class f: Membrane and cell surface proteins and peptides
Timeline for Class f: Membrane and cell surface proteins and peptides:
- Class f: Membrane and cell surface proteins and peptides first appeared (with stable ids) in SCOP 1.55
- Class f: Membrane and cell surface proteins and peptides appears in SCOPe 2.07