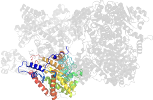
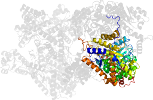
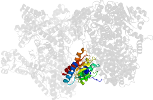Lineage for d2reqd1 (2req D:17-475)
- Root: SCOP 1.63

Class c: Alpha and beta proteins (a/b) [51349] (117 folds) 
Fold c.1: TIM beta/alpha-barrel [51350] (26 superfamilies)
contains parallel beta-sheet barrel, closed; n=8, S=8; strand order 12345678
the first seven superfamilies have similar phosphate-binding sites
Superfamily c.1.19: Cobalamin (vitamin B12)-dependent enzymes [51703] (3 families) 

Family c.1.19.1: Methylmalonyl-CoA mutase, N-terminal (CoA-binding) domain [51704] (1 protein) 
Protein Methylmalonyl-CoA mutase, alpha and beta subunits [51705] (1 species)
the subunits are clearly related but only one (alpha) is active
Species Propionibacterium freudenreichii, subsp. shermanii [TaxId:1744] [51706] (8 PDB entries) 
Domain d2reqd1: 2req D:17-475 [29643]
Other proteins in same PDB: d2reqa2, d2reqb2, d2reqc2, d2reqd2
complexed with b12, coa; mutant
Details for d2reqd1
PDB Entry: 2req (more details), 2.5 Å
PDB Description: methylmalonyl-coa mutase, non-productive coa complex, in open conformation representing substrate-free state
SCOP Domain Sequences for d2reqd1:
Sequence; same for both SEQRES and ATOM records: (download)
>d2reqd1 c.1.19.1 (D:17-475) Methylmalonyl-CoA mutase, alpha and beta subunits {Propionibacterium freudenreichii, subsp. shermanii}
tpttlslagdfpkateeqwerevekvlnrgrppekqltfaeclkrltvhtvdgidivpmy
rpkdapkklgypgvapftrgttvrngdmdawdvralhedpdekftrkaileglergvtsl
llrvdpdaiapehldevlsdvllemtkvevfsrydqgaaaealvsvyersdkpakdlaln
lgldpigfaalqgtepdltvlgdwvrrlakfspdsravtidaniyhnagagdvaelawal
atgaeyvralveqgftateafdtinfrvtathdqfltiarlralreawarigevfgvded
krgarqnaitswreltredpyvnilrgsiatfsasvggaesittlpftqalglpeddfpl
riarntgivlaeevnigrvndpaggsyyvesltrsladaawkefqeveklggmskavmte
hvtkvldacnaerakrlanrkqpitavsefpmigarsie
SCOP Domain Coordinates for d2reqd1:
Click to download the PDB-style file with coordinates for d2reqd1.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d2reqd1:
- d2reqd1 first appeared (with stable ids) in SCOP 1.55
- d2reqd1 appears in SCOP 1.61
- d2reqd1 appears in SCOP 1.65
- d2reqd1 appears in the current release, SCOPe 2.08
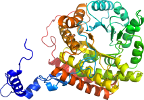
 View in 3D
View in 3D