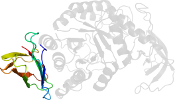
Lineage for d1bpl.1 (1bpl A:,B:193-393)
- Root: SCOP 1.69

Class c: Alpha and beta proteins (a/b) [51349] (136 folds) 
Fold c.1: TIM beta/alpha-barrel [51350] (31 superfamilies)
contains parallel beta-sheet barrel, closed; n=8, S=8; strand order 12345678
the first seven superfamilies have similar phosphate-binding sites
Superfamily c.1.8: (Trans)glycosidases [51445] (11 families) 
Family c.1.8.1: Amylase, catalytic domain [51446] (24 proteins)
members of the family may contain various insert subdomains
in alpha-amylases and closer relatives this domain is usually followed by a common all-beta domain
Protein Bacterial alpha-amylase [51447] (7 species) 
Species Bacillus licheniformis [TaxId:1402] [51448] (4 PDB entries) 
Domain d1bpl.1: 1bpl A:,B:193-393 [28703]
Other proteins in same PDB: d1bplb1
Details for d1bpl.1
PDB Entry: 1bpl (more details), 2.2 Å
PDB Description: glycosyltransferase
SCOP Domain Sequences for d1bpl.1:
Sequence; same for both SEQRES and ATOM records: (download)
>g1bpl.1 c.1.8.1 (A:,B:193-393) Bacterial alpha-amylase {Bacillus licheniformis}
lngtlmqyfewympndgqhwkrlqndsaylaehgitavwippaykgtsqadvgygaydly
dlgefhqkgtvrtkygtkgelqsaikslhsrdinvygdvvinhkggadatedvtavevdp
adrnrvisgehlikawthfhfpgrgstysdfkwhwyhfdgtdwdesrklnriykfqgkaX
ydylmyadidydhpdvaaeikrwgtwyanelqldgfrldavkhikfsflrdwvnhvrekt
gkemftvaeywqndlgalenylnktnfnhsvfdvplhyqfhaastqgggydmrkllnstv
vskhplkavtfvdnhdtqpgqslestvqtwfkplayafiltresgypqvfygdmygtkgd
sqreipalkhkiepilkarkq
SCOP Domain Coordinates for d1bpl.1:
Click to download the PDB-style file with coordinates for d1bpl.1.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1bpl.1:
- d1bpl.1 first appeared (with stable ids) in SCOP 1.55
- d1bpl.1 appears in SCOP 1.67
- d1bpl.1 appears in SCOP 1.71
- d1bpl.1 appears in the current release, SCOPe 2.08
 View in 3D
View in 3D