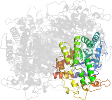
Lineage for d2fvma1 (2fvm A:2-56,A:441-541)
- Root: SCOPe 2.04

Class b: All beta proteins [48724] (176 folds) 
Fold b.92: Composite domain of metallo-dependent hydrolases [51337] (1 superfamily)
pseudobarrel; mixed sheet of 7 strand folded upon itself and "buckled" by two beta-turns
Superfamily b.92.1: Composite domain of metallo-dependent hydrolases [51338] (12 families) 
this domain is interrupted by the catalytic beta/alpha barrel domain
Family b.92.1.3: Hydantoinase (dihydropyrimidinase) [75044] (5 proteins) 
Protein Dihydropyrimidine amidohydrolase Pyd2 [141683] (2 species) 
Species Yeast (Saccharomyces kluyveri) [TaxId:4934] [141684] (3 PDB entries)
Uniprot Q9P903 2-56,441-541
Domain d2fvma1: 2fvm A:2-56,A:441-541 [134212]
Other proteins in same PDB: d2fvma2, d2fvmb2, d2fvmc2, d2fvmd2
automated match to d2ftya1
complexed with urp, zn
Details for d2fvma1
PDB Entry: 2fvm (more details), 2.45 Å
PDB Description: Crystal structure of dihydropyrimidinase from Saccharomyces kluyveri in complex with the reaction product N-carbamyl-beta-alanine
PDB Compounds: (A:) dihydropyrimidinaseSCOPe Domain Sequences for d2fvma1:
Sequence; same for both SEQRES and ATOM records: (download)
>d2fvma1 b.92.1.3 (A:2-56,A:441-541) Dihydropyrimidine amidohydrolase Pyd2 {Yeast (Saccharomyces kluyveri) [TaxId: 4934]}
piydliikngiictasdiyaaeiavnngkvqliaasidpslgsevidaegafitpXilpg
vsdadlviwypddskkeynskpklitnklmehncdytpfegieiknwprytivkgkivyk
egeilkenadgkylkrgksfmctpknewvtewrpkye
SCOPe Domain Coordinates for d2fvma1:
Click to download the PDB-style file with coordinates for d2fvma1.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d2fvma1:
- d2fvma1 first appeared in SCOP 1.73
- d2fvma1 appears in SCOPe 2.03
- d2fvma1 appears in SCOPe 2.05
- d2fvma1 appears in the current release, SCOPe 2.08
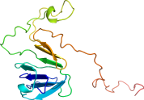
 View in 3D
View in 3D