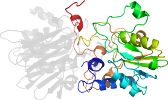
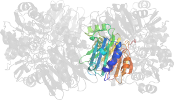
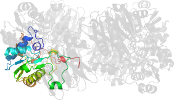
Lineage for d1gph32 (1gph 3:1-234)
- Root: SCOPe 2.08

Class d: Alpha and beta proteins (a+b) [53931] (396 folds) 
Fold d.153: Ntn hydrolase-like [56234] (2 superfamilies)
4 layers: alpha/beta/beta/alpha; has an unusual sheet-to-sheet packing
Superfamily d.153.1: N-terminal nucleophile aminohydrolases (Ntn hydrolases) [56235] (8 families) 
N-terminal residue provides two catalytic groups, nucleophile and proton donor
Family d.153.1.1: Class II glutamine amidotransferases [56236] (6 proteins)
has slightly different topology than other families do
Protein Glutamine PRPP amidotransferase, N-terminal domain [56239] (3 species) 
Species Bacillus subtilis [TaxId:1423] [56240] (2 PDB entries) 
Domain d1gph32: 1gph 3:1-234 [41814]
Other proteins in same PDB: d1gph11, d1gph21, d1gph31, d1gph41
complexed with amp, sf4
Details for d1gph32
PDB Entry: 1gph (more details), 3 Å
PDB Description: structure of the allosteric regulatory enzyme of purine biosynthesis
PDB Compounds: (3:) glutamine phosphoribosyl-pyrophosphate amidotransferaseSCOPe Domain Sequences for d1gph32:
Sequence; same for both SEQRES and ATOM records: (download)
>d1gph32 d.153.1.1 (3:1-234) Glutamine PRPP amidotransferase, N-terminal domain {Bacillus subtilis [TaxId: 1423]}
cgvfgiwgheeapqityyglhslqhrgqegagivatdgekltahkgqglitevfqngels
kvkgkgaighvryataggggyenvqpllfrsqnngslalahngnlvnatqlkqqlenqgs
ifqtssdtevlahlikrsghftlkdqiknslsmlkgayaflimtetemivaldpnglrpl
sigmmgdayvvasetcafdvvgatylrevepgemliindegmkserfsmninrs
SCOPe Domain Coordinates for d1gph32:
Click to download the PDB-style file with coordinates for d1gph32.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1gph32:
- d1gph32 first appeared (with stable ids) in SCOP 1.55
- d1gph32 appears in SCOPe 2.07

 View in 3D
View in 3D