Lineage for d1taea_ (1tae A:)
- Root: SCOPe 2.05

Class d: Alpha and beta proteins (a+b) [53931] (381 folds) 
Fold d.142: ATP-grasp [56058] (2 superfamilies)
Consists of two subdomains with different alpha+beta folds
shares functional and structural similarities with the PIPK and protein kinase superfamilies
Superfamily d.142.2: DNA ligase/mRNA capping enzyme, catalytic domain [56091] (6 families) 
has a circularly permuted topology
Family d.142.2.2: Adenylation domain of NAD+-dependent DNA ligase [56096] (2 proteins)
automatically mapped to Pfam PF01653
Protein Adenylation domain of NAD+-dependent DNA ligase [56097] (4 species)
contains additional, N-terminal all-alpha subdomain
Species Enterococcus faecalis [TaxId:1351] [118124] (9 PDB entries)
Uniprot Q837V6 5-317
Domain d1taea_: 1tae A: [112376]
complexed with na, nad, so4
Details for d1taea_
PDB Entry: 1tae (more details), 2.7 Å
PDB Description: Structural rearrangement accompanying NAD+ synthesis within a bacterial DNA ligase crystal
PDB Compounds: (A:) DNA ligase, NAD-dependentSCOPe Domain Sequences for d1taea_:
Sequence; same for both SEQRES and ATOM records: (download)
>d1taea_ d.142.2.2 (A:) Adenylation domain of NAD+-dependent DNA ligase {Enterococcus faecalis [TaxId: 1351]}
qpltltaattraqelrkqlnqysheyyvkdqpsvedyvydrlykelvdietefpdlitpd
sptqrvggkvlsgfekaphdipmyslndgfskedifafdervrkaigkpvayccelkidg
laislryengvfvrgatrgdgtvgenitenlrtvrsvpmrltepisvevrgecympkqsf
valneereengqdifanprnaaagslrqldtkivakrnlntflytvadfgpmkaktqfea
leelsaigfrtnperqlcqsidevwayieeyhekrstlpyeidgivikvnefalqdelgf
tvkaprwaiaykfppeeaetv
SCOPe Domain Coordinates for d1taea_:
Click to download the PDB-style file with coordinates for d1taea_.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1taea_:
- d1taea_ first appeared in SCOP 1.71
- d1taea_ appears in SCOPe 2.04
- d1taea_ appears in SCOPe 2.06
- d1taea_ appears in the current release, SCOPe 2.08
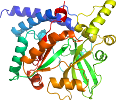
 View in 3D
View in 3D