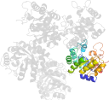Lineage for d1o66c1 (1o66 C:2-256)
- Root: SCOPe 2.06

Class c: Alpha and beta proteins (a/b) [51349] (148 folds) 
Fold c.1: TIM beta/alpha-barrel [51350] (33 superfamilies)
contains parallel beta-sheet barrel, closed; n=8, S=8; strand order 12345678
the first seven superfamilies have similar phosphate-binding sites
Superfamily c.1.12: Phosphoenolpyruvate/pyruvate domain [51621] (8 families) 

Family c.1.12.8: Ketopantoate hydroxymethyltransferase PanB [89503] (2 proteins)
automatically mapped to Pfam PF02548
Protein Ketopantoate hydroxymethyltransferase PanB [89504] (3 species)
dodecameric enzyme; a C-terminal helix exchange is observed in the M. tuberculosis enzyme but not in the E. coli enzyme
Species Neisseria meningitidis [TaxId:487] [102100] (2 PDB entries) 
Domain d1o66c1: 1o66 C:2-256 [92549]
Other proteins in same PDB: d1o66a2, d1o66b2, d1o66c2, d1o66d2, d1o66e2
structural genomics
complexed with gol
Details for d1o66c1
PDB Entry: 1o66 (more details), 1.75 Å
PDB Description: crystal structure of 3-methyl-2-oxobutanoate hydroxymethyltransferase
PDB Compounds: (C:) 3-methyl-2-oxobutanoate hydroxymethyltransferaseSCOPe Domain Sequences for d1o66c1:
Sequence, based on SEQRES records: (download)
>d1o66c1 c.1.12.8 (C:2-256) Ketopantoate hydroxymethyltransferase PanB {Neisseria meningitidis [TaxId: 487]}
itvntlqkmkaagekiamltayessfaalmddagvemllvgdslgmavqgrkstlpvslr
dmcyhtecvargaknamivsdlpfgayqqskeqafaaaaelmaagahmvkleggvwmaet
teflqmrgipvcahigltpqsvfafggykvqgrggkaqallndakahddagaavvlmecv
laelakkvtetvscptigigagadcdgqvlvmhdmlgifpgktakfvknfmqghdsvqaa
vrayvaevkaktfpa
Sequence, based on observed residues (ATOM records): (download)
>d1o66c1 c.1.12.8 (C:2-256) Ketopantoate hydroxymethyltransferase PanB {Neisseria meningitidis [TaxId: 487]}
itvntlqkmkaagekiamltayessfaalmddagvemllvgdslgmavqgrkstlpvslr
dmcyhtecvargaknamivsdlpfgayqqskeqafaaaaelmaagahmvkleggvwmaet
teflqmrgipvcahigltpqsvfakaqallndakahddagaavvlmecvlaelakkvtet
vscptigigagadcdgqvlvmhdmlgifpgktakfvknfmqghdsvqaavrayvaevkak
tfpa
SCOPe Domain Coordinates for d1o66c1:
Click to download the PDB-style file with coordinates for d1o66c1.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1o66c1:
- d1o66c1 first appeared in SCOP 1.67, called d1o66c_
- d1o66c1 was called d1o66c_ in SCOPe 2.05
- d1o66c1 appears in SCOPe 2.07
- d1o66c1 appears in the current release, SCOPe 2.08
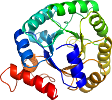
 View in 3D
View in 3D