Lineage for Protein: NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase
- Root: SCOP 1.67

Class c: Alpha and beta proteins (a/b) [51349] (130 folds) 
Fold c.3: FAD/NAD(P)-binding domain [51904] (1 superfamily)
core: 3 layers, b/b/a; central parallel beta-sheet of 5 strands, order 32145; top antiparallel beta-sheet of 3 strands, meander
Superfamily c.3.1: FAD/NAD(P)-binding domain [51905] (5 families) 

Family c.3.1.5: FAD/NAD-linked reductases, N-terminal and central domains [51943] (12 proteins)
duplication: both domains have similar folds and functions
most members of the family contain common C-terminal alpha+beta domain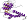
Protein NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase [82313] (1 species)
Species:

Xanthobacter sp., py2 [TaxId:35809] [82314] (2 PDB entries) - Domains for 1mo9:
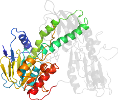
Domain d1mo9a1: 1mo9 A:2-192,A:314-383 [79341]
Other proteins in same PDB: d1mo9a3, d1mo9b3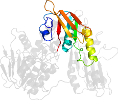
Domain d1mo9a2: 1mo9 A:193-313 [79342]
Other proteins in same PDB: d1mo9a3, d1mo9b3
Domain d1mo9b1: 1mo9 B:2-192,B:314-383 [79344]
Other proteins in same PDB: d1mo9a3, d1mo9b3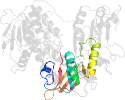
Domain d1mo9b2: 1mo9 B:193-313 [79345]
Other proteins in same PDB: d1mo9a3, d1mo9b3
complexed with fad, kpc
- Domains for 1mok:
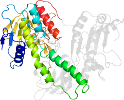
Domain d1moka1: 1mok A:2-192,A:314-383 [79347]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3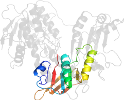
Domain d1moka2: 1mok A:193-313 [79348]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3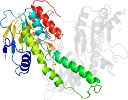
Domain d1mokb1: 1mok B:2-192,B:314-383 [79350]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3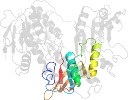
Domain d1mokb2: 1mok B:193-313 [79351]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3
Domain d1mokc1: 1mok C:2-192,C:314-383 [79353]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3
Domain d1mokc2: 1mok C:193-313 [79354]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3
Domain d1mokd1: 1mok D:2-192,D:314-383 [79356]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3
Domain d1mokd2: 1mok D:193-313 [79357]
Other proteins in same PDB: d1moka3, d1mokb3, d1mokc3, d1mokd3
complexed with fad
- Domains for 1mo9:
More info for Protein NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase from c.3.1.5: FAD/NAD-linked reductases, N-terminal and central domains
Timeline for Protein NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase from c.3.1.5: FAD/NAD-linked reductases, N-terminal and central domains:
- Protein NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase from c.3.1.5: FAD/NAD-linked reductases, N-terminal and central domains first appeared in SCOP 1.63
- Protein NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase from c.3.1.5: FAD/NAD-linked reductases, N-terminal and central domains appears in SCOP 1.65
- Protein NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase from c.3.1.5: FAD/NAD-linked reductases, N-terminal and central domains appears in SCOP 1.69
- Protein NADH-dependent 2-ketopropyl coenzyme M oxidoreductase/carboxylase from c.3.1.5: FAD/NAD-linked reductases, N-terminal and central domains last appears in SCOPe 2.07