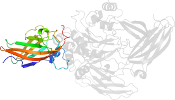
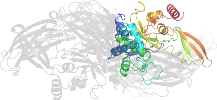
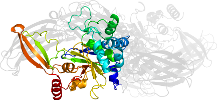
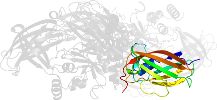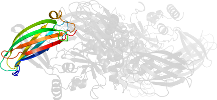Lineage for d1l9ma4 (1l9m A:141-461)
- Root: SCOPe 2.07

Class d: Alpha and beta proteins (a+b) [53931] (388 folds) 
Fold d.3: Cysteine proteinases [54000] (1 superfamily)
consists of one alpha-helix and 4 strands of antiparallel beta-sheet and contains the catalytic triad Cys-His-Asn
Superfamily d.3.1: Cysteine proteinases [54001] (24 families) 
the constitute families differ by insertion into and circular permutation of the common catalytic core made of one alpha-helix and 3-strands of beta-sheet
Family d.3.1.4: Transglutaminase core [54044] (3 proteins) 
Protein Transglutaminase catalytic domain [54045] (4 species) 
Species Human (Homo sapiens), TGase E3 [TaxId:9606] [75334] (9 PDB entries) 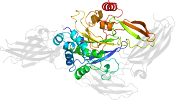
Domain d1l9ma4: 1l9m A:141-461 [73735]
Other proteins in same PDB: d1l9ma1, d1l9ma2, d1l9ma3, d1l9mb1, d1l9mb2, d1l9mb3
complexed with br, ca, cl
Details for d1l9ma4
PDB Entry: 1l9m (more details), 2.1 Å
PDB Description: three-dimensional structure of the human transglutaminase 3 enzyme: binding of calcium ions change structure for activation
PDB Compounds: (A:) Protein-glutamine glutamyltransferase E3SCOPe Domain Sequences for d1l9ma4:
Sequence; same for both SEQRES and ATOM records: (download)
>d1l9ma4 d.3.1.4 (A:141-461) Transglutaminase catalytic domain {Human (Homo sapiens), TGase E3 [TaxId: 9606]}
dsvfmgnhaereeyvqedagiifvgstnrigmigwnfgqfeedilsiclsildrslnfrr
daatdvasrndpkyvgrvlsaminsnddngvlagnwsgtytggrdprswdgsveilknwk
ksglspvrygqcwvfagtlntalrslgipsrvitnfnsahdtdrnlsvdvyydpmgnpld
kgsdsvwnfhvwnegwfvrsdlgpsyggwqvldatpqersqgvfqcgpasvigvregdvq
lnfdmpfifaevnadritwlydnttgkqwknsvnshtigryistkavgsnarmdvtdkyk
ypegsdqerqvfqkalgklkp
SCOPe Domain Coordinates for d1l9ma4:
Click to download the PDB-style file with coordinates for d1l9ma4.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1l9ma4:
- d1l9ma4 first appeared in SCOP 1.61
- d1l9ma4 appears in SCOPe 2.06
- d1l9ma4 appears in the current release, SCOPe 2.08
 View in 3D View in 3DDomains from other chains: (mouse over for more information) d1l9mb1, d1l9mb2, d1l9mb3, d1l9mb4 |