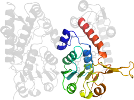Lineage for d1diba1 (1dib A:127-296)
- Root: SCOP 1.71

Class c: Alpha and beta proteins (a/b) [51349] (134 folds) 
Fold c.2: NAD(P)-binding Rossmann-fold domains [51734] (1 superfamily)
core: 3 layers, a/b/a; parallel beta-sheet of 6 strands, order 321456
The nucleotide-binding modes of this and the next two folds/superfamilies are similar
Superfamily c.2.1: NAD(P)-binding Rossmann-fold domains [51735] (12 families) 
Family c.2.1.7: Aminoacid dehydrogenase-like, C-terminal domain [51883] (11 proteins)
extra N-terminal helix displaces the C-terminal helix (following strand 6) from its usual position creating a family nicotineamide-binding site
Protein Methylenetetrahydrofolate dehydrogenase/cyclohydrolase [51894] (3 species)
the two-domain organization is similar to that of aminoacid dehydrogenases, but both domains are truncated
Species Human (Homo sapiens) [TaxId:9606] [51895] (4 PDB entries) 
Domain d1diba1: 1dib A:127-296 [30284]
Other proteins in same PDB: d1diba2, d1dibb2
Details for d1diba1
PDB Entry: 1dib (more details), 2.7 Å
PDB Description: human methylenetetrahydrofolate dehydrogenase / cyclohydrolase complexed with nadp and inhibitor ly345899
SCOP Domain Sequences for d1diba1:
Sequence, based on SEQRES records: (download)
>d1diba1 c.2.1.7 (A:127-296) Methylenetetrahydrofolate dehydrogenase/cyclohydrolase {Human (Homo sapiens)}
ltsinagrlargdlndcfipctpkgcleliketgvpiagrhavvvgrskivgapmhdlll
wnnatvttchsktahldeevnkgdilvvatgqpemvkgewikpgaividcginyvpddkk
pngrkvvgdvaydeakerasfitpvpggvgpmtvamlmqstvesakrfle
Sequence, based on observed residues (ATOM records): (download)
>d1diba1 c.2.1.7 (A:127-296) Methylenetetrahydrofolate dehydrogenase/cyclohydrolase {Human (Homo sapiens)}
ltsinagrlargdlndcfipctpkgcleliketgvpiagrhavvvgrskivgapmhdlll
wnnatvttchsktahldeevnkgdilvvatgqpemvkgewikpgaividcginykvvgdv
aydeakerasfitpvpggvgpmtvamlmqstvesakrfle
SCOP Domain Coordinates for d1diba1:
Click to download the PDB-style file with coordinates for d1diba1.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1diba1:
- d1diba1 first appeared (with stable ids) in SCOP 1.55
- d1diba1 appears in SCOP 1.69
- d1diba1 appears in SCOP 1.73
- d1diba1 appears in the current release, SCOPe 2.08
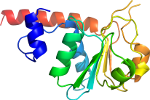
 View in 3D
View in 3D