Lineage for d1piia1 (1pii A:255-452)
- Root: SCOP 1.73

Class c: Alpha and beta proteins (a/b) [51349] (141 folds) 
Fold c.1: TIM beta/alpha-barrel [51350] (33 superfamilies)
contains parallel beta-sheet barrel, closed; n=8, S=8; strand order 12345678
the first seven superfamilies have similar phosphate-binding sites
Superfamily c.1.2: Ribulose-phoshate binding barrel [51366] (6 families) 

Family c.1.2.4: Tryptophan biosynthesis enzymes [51381] (3 proteins) 
Protein N-(5'phosphoribosyl)antranilate isomerase, PRAI [51382] (3 species) 
Species Escherichia coli [TaxId:562] [51383] (1 PDB entry)
merged in bifunctional enzyme with IPG synthase
Domain d1piia1: 1pii A:255-452 [28559]
Other proteins in same PDB: d1piia2
complexed with po4
Details for d1piia1
PDB Entry: 1pii (more details), 2 Å
PDB Description: three-dimensional structure of the bifunctional enzyme phosphoribosylanthranilate isomerase: indoleglycerolphosphate synthase from escherichia coli refined at 2.0 angstroms resolution
PDB Compounds: (A:) n-(5'phosphoribosyl)anthranilate isomeraseSCOP Domain Sequences for d1piia1:
Sequence; same for both SEQRES and ATOM records: (download)
>d1piia1 c.1.2.4 (A:255-452) N-(5'phosphoribosyl)antranilate isomerase, PRAI {Escherichia coli [TaxId: 562]}
genkvcgltrgqdakaaydagaiygglifvatsprcvnveqaqevmaaaplqyvgvfrnh
diadvvdkakvlslaavqlhgneeqlyidtlrealpahvaiwkalsvgetlparefqhvd
kyvldngqggsgqrfdwsllngqslgnvllagglgadncveaaqtgcagldfnsavesqp
gikdarllasvfqtlray
SCOP Domain Coordinates for d1piia1:
Click to download the PDB-style file with coordinates for d1piia1.
(The format of our PDB-style files is described here.)
(The format of our PDB-style files is described here.)
Timeline for d1piia1:
- d1piia1 first appeared (with stable ids) in SCOP 1.55, called d1pii_2
- d1piia1 was called d1pii_2 in SCOP 1.71
- d1piia1 appears in SCOP 1.75
- d1piia1 appears in the current release, SCOPe 2.08
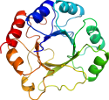
 View in 3D
View in 3D